CURRENT NEED: Medical countermeasures (MCMs) are an essential component for the protection of the warfighter, needed to ensure our warfighters are capable to fight and win wars. MCMs are a critical component of integrated layered defense that provide medical prophylaxis and therapeutic solutions to sustain combat power. Unfortunately, MCMs historically require significant time (greater than 5 years) to develop and field due to challenging technical developments, regulatory prerequisites, transitions through stove-piped developmental activities, and needs for industry expertise and partnership. As a result of these timelines and in light of a rapidly evolving threat environment, countermeasures developed today against known threats may not be relevant on the battlefield when they are ready. Readiness must now address not only known, but emerging and wholly unanticipated biological and chemical threat agents.
SOLUTION APPROACH: A solution to this challenge resides in the implementation of an agile and adaptive acquisition strategy using Capability Portfolio Management (CPM) and implementing a System of System (SoS) approach to deliver integrated and innovative risk-informed solutions1. The impetus of the SoS approach is to deliver at speed to both emerging and changing threat spaces, as well as maintain technological relevancy to pace with the dynamic and expanding threat.
CPM focuses on investment areas that work to deliver solutions for desired outcomes given a capability area (eg. Chemical prophylaxis medical countermeasures). This focus leverages the entire product development spectrum from Science & Technology (S&T) through Advanced Development (AD) to focus on a capability and allow the capability need to drive the development of the solution set. Traditionally stove-piped development was used to deliver segmented capability towards specific capability gaps. The inherent barriers in this approach are removed through the application of a SoS approach within CPM that establishes an integrated acquisition development process to deliver on desired capability outcomes. This process iteratively integrates S&T, Integrated Master List (IML), and Acquisition Master List (AML) activities across the MCM capability development spectrum through the incorporation of acquisition reform tenants. Through iterative integration the enterprise approach achieves rapid risk reduction during development, accelerated delivery of needed MCM capabilities to the warfighter, and simultaneous development of enduring platforms that enable the acceleration of future demands and technological relevancy to stay abreast of warfighter needs and evolving threats.
When these tenants are applied in the SoS approach MCM capability development, timelines are decreased by leveraging S&T pipelines and IML programs that enable rapid prototyping, eventually providing a natural on-ramp for technology integration into programs of record (PoR)2. Through this acceleration PoR’s Future Years Defense Program (FYDP) investment needs are reduced due to the early risk reduction and transition of MCM products further along in the MCM capability development lifecycle.
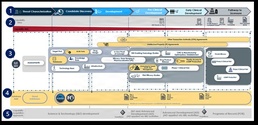
The second photo depicts the entire SoS across the MCM development spectrum, starting with a signal and ending with pathway to Food and Drug Administration (FDA) licensure. The approach presents some clear improvements on the historical stove pipe development process, mainly insofar as providing interconnected activities with feedback loops, and affording the opportunity to accelerate development by inserting it an appropriate phase of MCM development. The figure highlights data injects and pulls from enabling capabilities across and within development activities, and the need for specific focus on flexible contracting actions that are capable of transition across phases of development. An important facet of the SoS is the delivery of incremental ”Capability Wins” along the total development pathway that inform future risk-based decisions, and prepare the warfighter for potential emergency response (e.g. Expanded Access Protocols (EAPs), stockpiling, etc.) In the case of chemical prophylaxis MCM development, the determination to initiate the system begins with the identification of a threat or gap. At this point multiple phases of the SoS can engage to mitigate development risk, by targeting products with varying levels of technical readiness for development and inserting them into the pathway at the appropriate stage. Once development is initiated the follow-on development activity monitors for transition and accepts when ready. Once received, the next activity is able to return or advance the capability under development based on risk-based decisions in development. The developing activities submit data transitions that incrementally provide Capability Wins. The result is multiple capabilities brought through varying stages of development to improve medical readiness and response coverage against the chemical prophylaxis MCM need.
CONCLUSION: New approaches are critical to accelerating and delivering live-saving medical countermeasures to the warfighter at time-of-need. The outlined approach implements acquisition reforms while inserting innovation in a System of Systems approach that supports development within a capability area. This holistic CPM approach provides increased understanding across capability development that enables purposeful risk-based decision making to accelerate MCM delivery to the warfighter in support of integrated layered defense. Through iterative data sharing, agile program management, and blended acquisition approaches, the established SoS produces a kinetic system that delivers incremental capability wins to the warfighter, reducing development risks across time, as well as steady investment in less mature technologies to jumpstart the next demand and prepare for potential future MCM needs.
REFERENCES: 1. DOD Directive 7045.2 Capability Portfolio Management 2. ‘The Need for Speed: Streamlining Procurement for Effective Execution and Delivery Act’
About the writers: Elizabeth (Liz) Smith currently serves as the Deputy Director for the Advanced Technology Products (ATP) program at the Joint Project Manager for Chemical, Biological, Radiological, and Nuclear Defense’s Enabling Biotechnologies. Ms. Smith brings a wealth of technical experience from industry, homeland security, as well as over 10 years supporting various initiatives across the Chemical Biological Defense enterprise.
Rachel Campbell de Bautista is a highly accomplished Chemical and Biological Defense enterprise program manager with a proven record of driving highly complex technical program in the artificial intelligence/machine learning and medical countermeasure development sector. Ms. Campbell de Bautista currently serves as theDeputy Director for the Data & Technology Integration for Readiness at the Joint Project Manager for Chemical, Biological, Radiological, and Nuclear Defense’s Enabling Biotechnologies.