Humanoid Cells Testing
Defense Threat Reduction Agency's Chemical and Biological Technologies Department
Courtesy Story
Date: 04.27.2020
Posted: 04.27.2020 11:47
News ID: 368549
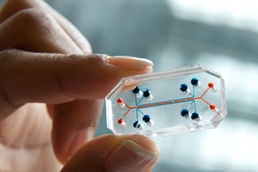
Research studies typically take months or years to yield data on how a new chemical and biological threat agent can hurt the human body. However, scientists have learned that combining select state-of-the-art assessment methods can yield data in far less time — sometimes within hours — making it possible to predict what an emerging chemical or biological threat agent can do to a warfighter before the warfighter encounters it. The Defense Threat Reduction Agency (DTRA) supported the U.S. Army’s Combat Capability Development Command, Chemical, and Biological Center (CCDC CBC) to select and develop predictive toxicology methods. The predictive methods combine computer-based (in silico) models and relatively quick laboratory (in vitro) methods. The methods rapidly generate specific data, such as where exactly in the human body an emerging agent will cause damage.
Through DTRA’s support, CCDC CBC scientists are developing and validating the organ-on-a-chip technology — which can use animal cells or cells derived from humans — to assess an agent’s toxicity to a human organ. They are also working with the Defense Advanced Research Projects Agency to transition state-of-the-art predictive in vitro assays to CCDC CBC’s toxicology program, so organ-on-a-chip is just one of many in vitro capabilities being developed and used.1 Scientists at CCDC CBC are primarily concerned with cardiac and central nervous system effects of some emerging threats, so they also evaluate the effects of emerging threats on live zebra fish. Zebra fish are less expensive to maintain and breed than other vertebrate small animals, such as mice, and zebra fish assays provide threat-toxicity-effects data that are also accurate for understanding the effects in humans. Whether the data are extrapolative or are directly gathered from human cells, the in vitro tests — when combined with in silico data — help scientists understand how agents affect various biological systems, thus providing information other scientists can use to develop medical countermeasures.
Predictive toxicology is proving, in many cases, to more accurately characterize a person’s biological response to an exposure to a chemical or biological agent than traditional animal studies do. There are several reasons for this:
- Predictive in vitro methods often use human cells, which can more accurately identify how an agent physically or chemically behaves in a person. Otherwise, data derived from animal studies are used and require scientists to extrapolate from these data to understand and estimate the human response.
- Scientists can often tailor predictive in vitro methods to mimic the conditions in which a person could be exposed to an agent. Scientists can test a range of exposure levels a person may encounter and identify how human cells react at those exposures.
- With access to information from past chemistry, toxicology, and pathology studies, scientists can combine the data with results from predictive in silico methods to better understand how an agent may injure the human body. For example, by using well-understood chemistry and the molecular structure of the emerging threat along with human organ data, scientists can predict how the agent could injure the human body.
Using in silico and in vitro predictive methods together can help scientists identify key biological responses to unknown chemical and biological threat agents. Typically, in silico models help scientists focus on a compound’s reactive nature, enabling scientists to select the most relevant in vitro toxicology tests to confirm and quantify the response. Because in vitro assays can use human-derived cells, scientists will not have to infer how humans will respond to a threat agent based on data from animal studies.
The new predictive toxicology methods will change how DoD evaluates the physiological effects of new and emerging chemical and biological threat agents at a fraction of the cost and time compared to traditional toxicology methods. Predictive toxicology methods offer DoD the capability to rapidly identify how an emerging chemical and biological threat agent will hurt the warfighter’s body. Warfighters, in turn, will know the dangers of the emerging threat agent before encountering it on the battlefield.
1 An assay is a scientific method or test used to examine or analyze a substance in order to determine its toxicity and better understand how it behaves. An in vitro assay is a test done outside of an animal or person.
POC: Donald Cronce, Ph.D; donald.t.cronce.civ@mail.mil
