The last case of smallpox in the United States was reported more than 65 years ago, and in 1977 the disease was eradicated due to the development of a vaccine. Vaccinations such as this are critical to protecting our warfighters and the public from biological threats, but many threats still lack countermeasures. Melioidosis, a Centers for Disease Control category B agent, claimed 89,000 lives in 2015 alone, which has prompted researchers from the Defense Threat Reduction Agency’s Chemical and Biological Technologies Department to lead development of the first vaccine for the deadly disease.
Melioidosis is caused by the bacterium Burkholderia pseudomallei, via inhalation of contaminated dust or water, or contact with contaminated soil, especially through skin abrasions. The signs and symptoms of the disease can vary greatly and may mimic those of tuberculosis or common forms of pneumonia.
Historically, the disease is associated with a high mortality rate due to its quick progression, the difficulty of diagnosis, and the inherent resistance of the bacteria to several antibiotics. Both the intrinsic drug resistance and the intracellular lifestyle make successful antibiotic treatment difficult, lengthy and intensive, often involving intravenous therapy followed by a months-long eradication phase with oral antibiotics.
In addition to naturally occurring infections, B. pseudomallei is considered to be a high risk for terrorism use due to the ease of acquiring strains from the environment. The organism is endemic in the soil and water of many tropical areas across Southeast Asia, the Indian subcontinent, northern Australia and parts of Africa, South America and the Caribbean. Given this threat and the lengthy treatment regimen with suboptimal outcomes, there is significant need to develop an effective countermeasure.
Paul J. Brett, Ph.D., and Mary N. Burtnick, Ph.D., Associate Professors at the University of Nevada and the University of South Alabama recently published the research in the Infection and Immunity article “Development of Subunit Vaccines That Provide High-Level Protection and Sterilizing Immunity against Acute Inhalational Melioidosis.” Their work found a subunit vaccine candidate that generated sterilizing immune responses in mice.
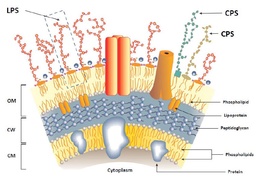
B. pseudomallei expresses a variety of structurally conserved protective antigens, including cell-surface polysaccharides, cell-associated proteins and secreted proteins. The capsular polysaccharide (CPS) is expressed by all known virulent isolates of B. pseudomallei and therefore an attractive antigen for vaccine development.
Researchers tested CPS, in combination with hemolysin co-regulated protecin as a second antigen, because it is highly conserved among B. pseudomallei isolates. Recently tested in mice, this two-part vaccine formulation stimulates both humoral and cellular immune responses to provide mice with a high-level protection and, importantly, sterilizing immunity, against an acute inhalational challenge of melioidosis.
After aerosol challenge with a high dose of B. pseudomallei, 100 percent of the mice immunized with the conjugate vaccine survived the full 35-day study. In contrast, all unvaccinated mice succumbed to disease by day eight. Remarkably, 70 percent of the survivors had no culturable bacteria in their lungs, livers or spleens, indicating that the vaccine formulation had generated sterilizing immune responses. To date, this is the highest level of protection conferred by a subunit vaccine against an acute inhalational challenge of B. pseudomallei.
Collectively, these studies support the rationale for developing multivalent subunit vaccines to immunize against disease caused by B. pseudomallei. Due to the high level of immunity demonstrated during the acute inhalational challenge, DTRA CB aims to develop life-saving vaccines for both the warfighter and public health against naturally occurring and engineered biological agents.