Similar to a classic board game, solving the mystery of “who done what, with what and where” can be a game of chance. Much is the case for a physician trying to distinguish between a bacterial or viral infection solely on physical symptoms, as many present similarly. However, deployed warfighters may not have the option to transport cumbersome diagnostic equipment, reducing proper diagnosis to deductive reasoning. Quickly differentiating between infections would allow warfighters to receive proper care as quickly as possible.
If a deployed warfighter becomes infected with an unknown biological threat agent, providing the appropriate course of treatment can be the difference between readiness and disaster. Viral infections are impervious to antibiotic drugs, so rapidly identifying the cause is vital for treatment.
As a part of its Host-Based Biomarker Discovery program, the Defense Threat Reduction Agency’s Chemical and Biological Technologies Department is tackling this problem by developing the ImmunoPOC™, a benchtop point-of-care diagnostic device. The device can differentiate between bacterial and viral infections within 15 minutes.
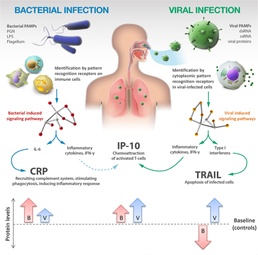
The ImmunoPOC™, in development by MeMed Diagnostics LTD., examines the combination of expression levels of three protein biomarkers found in blood that fluctuate depending on the type of infection. Specifically, the levels of the first biomarker, C-reactive protein, increase in the case of bacterial infections.
The levels of the second biomarker, interferon gamma induced protein-10, greatly increases with viral and less so in bacterial infections. Finally, the levels of the third biomarker, tumor necrosis factor-related apoptosis-inducing ligand, increases in the case of viral and decreases in bacterial infections.
Preliminary studies show 90 percent accuracy when examining this combination of biomarkers to distinguish between the two.
The potential use for the ImmunoPOC™ is wide-ranging. Designed for easy transport, the device can be set up in deployed environments. This ease of use will allow accurate test results to aide in timely and appropriate treatment while mitigating disease transmission to fellow warfighters. Further, the device will aide in combatting the proliferation of antimicrobial resistance bacteria by preventing antibiotic misuse through quick identification of viral infections and provide critical force health-protection information to decision makers. The ImmunoPOC™ will allow warfighters to receive the care they need when they need it, increasing battlefield readiness and effectiveness.
POC: Nathan Adams, Ph.D.; nathan.w.adams10.civ@mail.mil