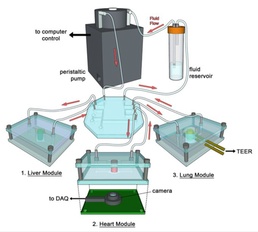
Currently, a critical need exists for an improved modeling system that can accurately predict the effects of drugs on chemical and biological agents in the human body. DTRA CB’s Integrated Organoid Testing System (INGOTS) answers that need by merging several key technologies to mimic human response to therapeutics.
The effort, led by Dr. Anthony Atala of Wake Forest Institute for Regenerative Medicine, is using human cells in proportion to in vivo counterparts, 3-D bioprinting, and microfluidic devices to develop a differentiated state and simulate the human circulator system. In addition, an air-liquid interface simulates lung response while real-time biosensors detect toxicity of the challenge agents.
In recent studies, INGOTS demonstrated prolonged viability and maintenance of a differentiated state which permits real-time assessment of relevant biomarkers.
Researchers first tested the liver-on-a-chip device, as liver toxicity and metabolism are responsible for many failures in drug development. The device accurately mimicked human response exhibiting sustained levels of albumin, a vital blood protein and urea, a molecule produced in the liver.
Similarly, in response to exposure to acetaminophen, the INGOT successfully replicated response observed by animal and human exposure, producing biomarkers indicative of cell damage.
Partial reversal of acetaminophen-induced damage by an anti-oxidant N-acetyl cysteine was also demonstrated, indicating the dynamic response to toxic molecules using real-time assessment in systems using human cell-derived organoids.
Further, important criteria indicative of the maintenance of a differentiated state include relevant organotypic responses to endogenous hormones. The cardiac organoid response to epinephrine and its reversal by metoprolol, a beta-blocker, is visible.
Finally, in the presence of liver organoids, metabolism of the beta-blocker propanol allows an increase in heart rate driven by the epinephrine. Thus, the relevance to clinical utility of such systems to reflect maintenance of an advanced differentiated state allows physiologically relevant responses, which is not possible in conventional 2-D models.
In another trial, researchers demonstrated the utility of INGOTS in lung metabolism in the evolution of cardiotoxic metabolites using three integrated organoid system models (liver-lung-cardiac) with the drug bleomycin, which is commonly used for cancer treatment. Alone, bleomycin does not appear to be toxic to the heart. However, in the presence of a lung organoid, the evolution of cytokines including IL-8 and IL-1β reveal the specific molecules involved in cardiotoxicity which are liberated by the lung.
Bleomycin and other cancer chemotherapeutics are indeed toxic to the heart, and these model systems allow investigators to dissect the chemical messengers responsible for this process. The key attributes of long-term maintenance of viability, differentiated state and metabolic competence allows these systems to be investigated further as tools in risk-mitigation and predictive toxicology.
Currently under evaluation by U.S. and European Union regulatory authorities as supplementary toxicology assessment tools, INGOTS aims to replace animal testing by accurately mimicking human physiologic and metabolic responses. Advances in predictive toxicology can speed the development of new therapeutics and vaccines to protect warfighters from the evolving threat of naturally occurring or engineered biological weapons.
For more information, view the Scientific Reports article, “Multi-Tissue Interactions in an Integrated Three-Tissue Organ-on-a-Chip Platform.”
POC: Dale E. Taylor; dale.e.taylor4.civ@mail.mil